pH Titration Curves 'Idealized':
To date the equivalence point of an acid base reaction
has been determined using an indicator. In this experiment we are
going to monitor the changes in pH that occurs during the titration of
a weak polyprotic acid with a strong base. At the equivalence point
one should expect to see a dramatic change in pH as the solution goes from
acidic to strongly basic.
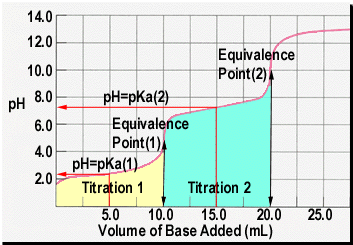 Depicted on the left is an idealized pH titration curve for a weak diprotic
acid. The first thing that you should notice is that there are two
regions where we see a significant pH change. These, if you wish,
correspond to two separate titrations. Titration 1 is the reaction
of the first proton with the base (in this case sodium hydroxide).
Depicted on the left is an idealized pH titration curve for a weak diprotic
acid. The first thing that you should notice is that there are two
regions where we see a significant pH change. These, if you wish,
correspond to two separate titrations. Titration 1 is the reaction
of the first proton with the base (in this case sodium hydroxide).
H2X(aq) + NaOH(aq) = NaHX(aq) + H2O(l)
NaHX(aq) + NaOH(aq) = Na2X(aq) + H2O(l)
H2X(aq) + 2 NaOH = Na2X(aq) + 2 H2O(l)
H2X(aq) + H2O(l) ®
HX- + H3O+
Ka = [H3O+][HX-]/[H2X]
or written in another way
[H3O+] = Ka{[H2X]/[HX-]}
[H3O+] = Ka{1/2[H2X]initial/[1/2H2X]initial}
[H3O+] = Ka
The first acid that you will be following today is
citric acid which is an acid that falls into the idealized category.
You should see three areas where the pH undergoes significant changes and
should be able to determine the three Ka values for citric acid and compare
the result to the three known values given below.
H3C6H5O7(aq)
+ H2O(l) <=>
H2C6H7O7-
+ H3O+ Ka1
= 7.4x10-3 @ 25oC
H2C6H5O7-
+ H2O(l) <=>
HC6H6O72-
+ H3O+ Ka2 = 1.7x10-5
@ 25oC
HC6H5O72-
+ H2O(l) <=>
C6H5O73-
+ H3O+ Ka3
= 4.0x10-7 @ 25oC
pH Titration Curves 'The Real World':
In reality, many polyprotic acids only show one discernable equivalence point! The vast majority of the time, this corresponds to final equilibrium. If this is the case then, all the other equivalence points can be determined by knowing what type of polyprotic acid one is dealing with, i.e., diprotic or tri protic. For a triprotic acid, the other two equivalence points should correspond to 1/3 and 2/3 of the volume of the base required to reach the final one and thus one can still determine the Ka values. Note, however, I did say the vast majority of the time. How one knows how to determine whether the observed equivalence point equals the removal of the final proton I leave for you to explore! [Hint: what is the pH at the 1/2 equivalence point of a titration of a polyprotic acid equal to]
The second acid that you will be looking at in this lab is phosphoric acid, a triprotic acid whose Ka values are given below.
H3PO4(aq) + H2O(l) <=>
H2PO4-
+ H3O+ Ka1 = 7.5x10-3 @ 25oC
H2PO4- + H2O(l)
<=> HPO42-
+ H3O+ Ka2= 6.2x10-8 @ 25oC
HPO42- + H2O(l) <=>
PO43-
+ H3O+ Ka3= 3.6x10-13 @ 25oC
Experimental Procedure:
Your TA will also demonstrate the best set up for this experiment.
- Using a graduated cylinder, place ~ 20mL of the ~0.02M citric acid into a small beaker. If necessary add distilled water such that the tip of the pH probe is covered.
- Fill your buret with the ~0.02M NaOH solution. Record the exact molarity of this solution. Record the initial buret reading. Remember that this corresponds to 0.00mL of NaOH added.
- Record the initial pH of the Citric acid.
- Carefully add the NaOH recording the volume of NaOH required to effect a pH change of 0.2. Continue this process until the pH reaches 12.
- Plot a graph of 'pH' versus 'Volume of NaOH" added and from this graph determine:
- The Ka values for citric acid.
-
The exact concentration of the citric acid.
- Repeat steps one through five using the ~0.02M phosphoric acid and determine the Ka values for phosphoric acid and the exact molarity of the phosphoric acid solution.
Write-up:
In the discussion portion of
your write up, be sure to address the correlation between the Ka values
that you obtained and those given to you in this procedure. Address
any unusual problems that you encountered.